Our Products
- SOLID TUMOUR
ONCODEcipher
SERIES - BLOOD CANCERS
ONCODEduce
SERIES - RISK
ASSESSMENT
LIQUID10
Liquid biopsy NGS panel targeting 10 actionable genes for mutations & fusions commonly mutated in cancer especially lung & colon cancer to provide treatment options & prognostic indications.
LIQUID100
Liquid biopsy NGS panel targeting >100 actionable genes in cancers for mutation, fusion and SNP to provide treatment options & prognostic indications.
HOMOLOGOUS
RECOMBINATION
DEFICIENCY
(HRD)
NGS panel to assess the status
of HRD through the detection of BRCA1/2 gene mutation AND determination of the genomic instability.
TISSUE
PREMIUM
NGS panel targeting mutation and fusion, MSI (PCR & Fragment analysis), PD-L1 (IHC) and blood germline NGS panel targeting genes associated with hereditary cancer predisposition, enabling the identification of somatic mutations as well as inherited cancer risks in individuals.
TISSUE10
FFPE NGS panel targeting 17 actionable genes for
mutations & fusions commonly mutated in cancers to provide treatment options & prognostic indications.
CANCER
HEREDITARY
Blood germline NGS panel targeting 31 genes
associated with hereditary cancer predisposition.
ONCODEcipher Lung Premium
FFPE DNA & blood panel targeting relevant and actionable genes commonly mutated in lung cancer to provide treatment options & prognostic indications.
ONCODEcipher Breast & Ovarian Premium
FFPE DNA & blood panel targeting relevant and actionable genes commonly mutated in breast & ovarian cancer to provide treatment options & prognostic indications.
ONCODEcipher Breast & Ovarian Liquid Premium
Non-invasive cell free circulating tumor DNA (ctDNA) panel that delivers information on a variety of treatment/prognosis informative mutations in the genes which are known to play a role in solid tumors using only the blood of the patients.
ONCODEcipher Colon & Endometrial Premium
FFPE DNA & blood panel targeting 67 relevant genetic mutations in colon and endometrial cancer AND microsatellite instability (MSI) status to provide treatment options & prognostic indications.

ONCODEcipher Pancreatic & Prostate Premium
FFPE DNA & blood panel targeting relevant and actionable genes commonly mutated in pancreatic and prostate cancer to provide treatment options & prognostic indications.
ONCODEcipher Tissue Premium
FFPE DNA & blood panel targeting relevant and actionable genes commonly mutated in cancerous cell tissue to provide treatment options & prognostic indications.

ONCODEcipher Liquid Premium
Non-invasive cell free circulating tumor DNA (ctDNA) panel that delivers information on a variety of treatment/prognosis informative mutations in the genes which are known to play a role in solid tumors using only the blood of the patients.

ONCODEcipher Liquid Premium Plus
Non-invasive cell free circulating tumor DNA (ctDNA) panel that delivers information on a variety of treatment/prognosis informative mutations in the genes which are known to play a role in solid tumors using only the blood of the patients
EOSINOPHILIA
FUSION
NGS panel to detect all fusions/rearrangements in PDGFRA (including FIP1L1::PDGFRA), PDGFRB, FGFR1, BCR, ABL1, FLT3, ETV6 and
JAK2 (including PCM1::JAK2) & mutation in KIT.
MUTATION
37-GENE
NGS panel targeting 37 gene mutations allows a broader exploration of the genes implicated in MPN & AML/MDS.
FUSION
87-GENE
NGS panel targeting 87
gene fusions/ rearrangements implicated in MPN, AML, B-ALL & T-ALL.
AML COMBO PLUS
Comprehensive NGS
panel for AML detecting mutation (23 genes) and
fusion (11 genes)
commonly implicated in AML to provide possible prognostic, therapeutic and diagnostic options.
ABL1 KINASE
DOMAIN
MUTATION
NGS panel designed to detect TKI resistance mutations in ABL1 kinase domain (amino acid positions 244-396)
and all possible BCR::ABL1 fusions.
MPN PCR PANEL
PCR-based test for the detection of mutations in JAK2 V617F & exon 12, CALR, MPL W515K/L/A & S505N.
BRAF V600E
QUALITATIVE
PCR
PCR assay to detect the presence of BRAF V600E variant for assessment of Hairy Cell Leukemia.
MYD88 L265P
QUALITATIVE
PCR
PCR assay to detect the
presence of MYD88 L265P somatic variants for diagnostic of
CD5 negative B-NHL and DLBCL.

Cancer Hereditary NGS Panel
PRODUCT DESCRIPTION
NGS panel specifically designed to target 31 germline genes known to be associated with hereditary cancer predisposition. This panel detects genetic variations in these genes, enabling the identification and assessment of potential inherited cancer risks in individuals.
Highlighted Genes:
Germline BRCA1&2, BARD1, PALB2, CHEK2, TP53, MLH1, MSH2, MSH6, PMS2, PTEN, STK11, EPCAM etc.
Alterations Detected:
SNVs, InDels, Large Genomic Rearrangements, Large Deletion/Duplications and CNVs.
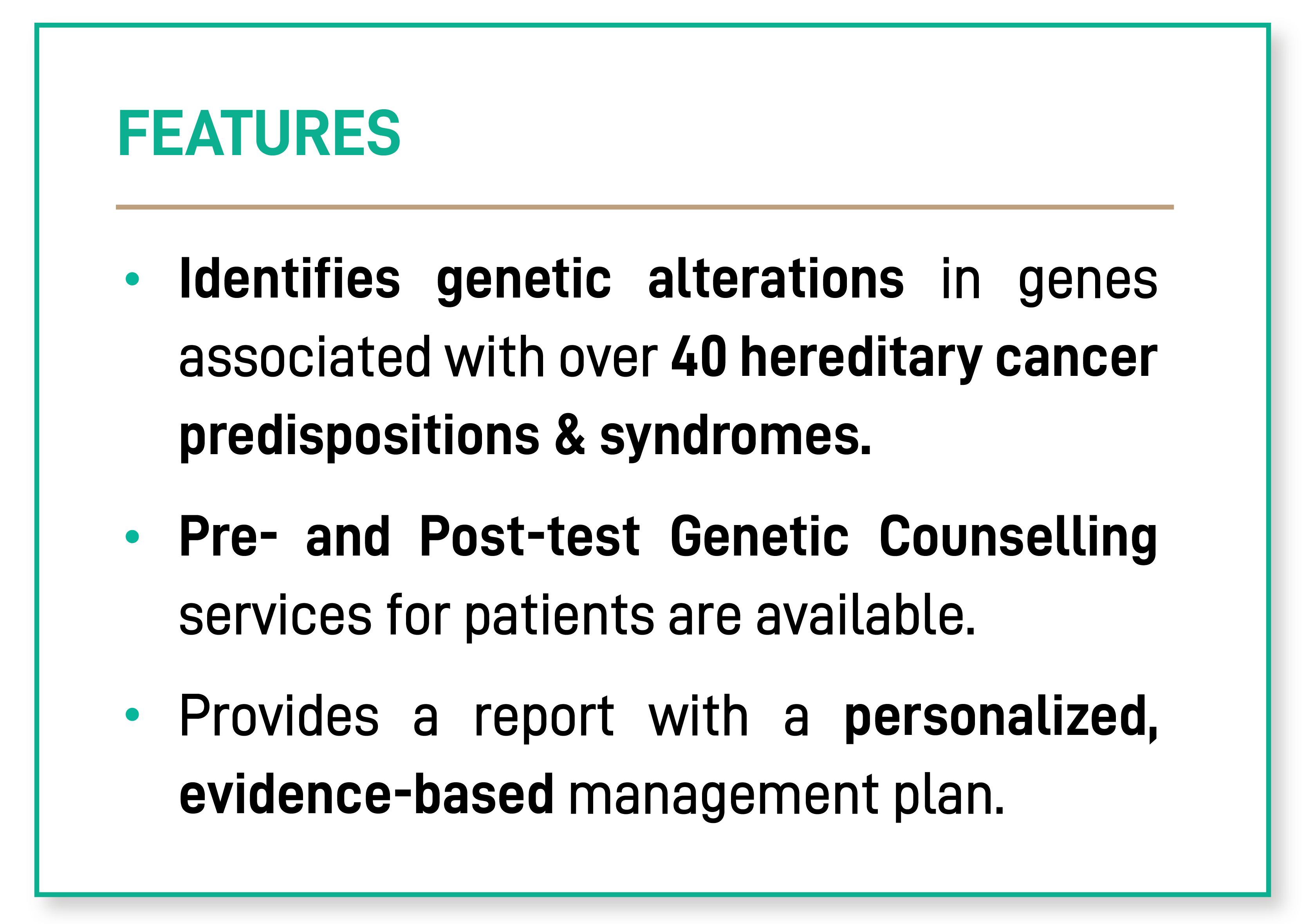
FEATURES
Identifies genetic alterations in genes associated with over 40 hereditary cancer predispositions & syndromes.
Pre- and Post-test Genetic Counselling services for patients are available.
Provides a report with a personalized, evidence-based management plan.
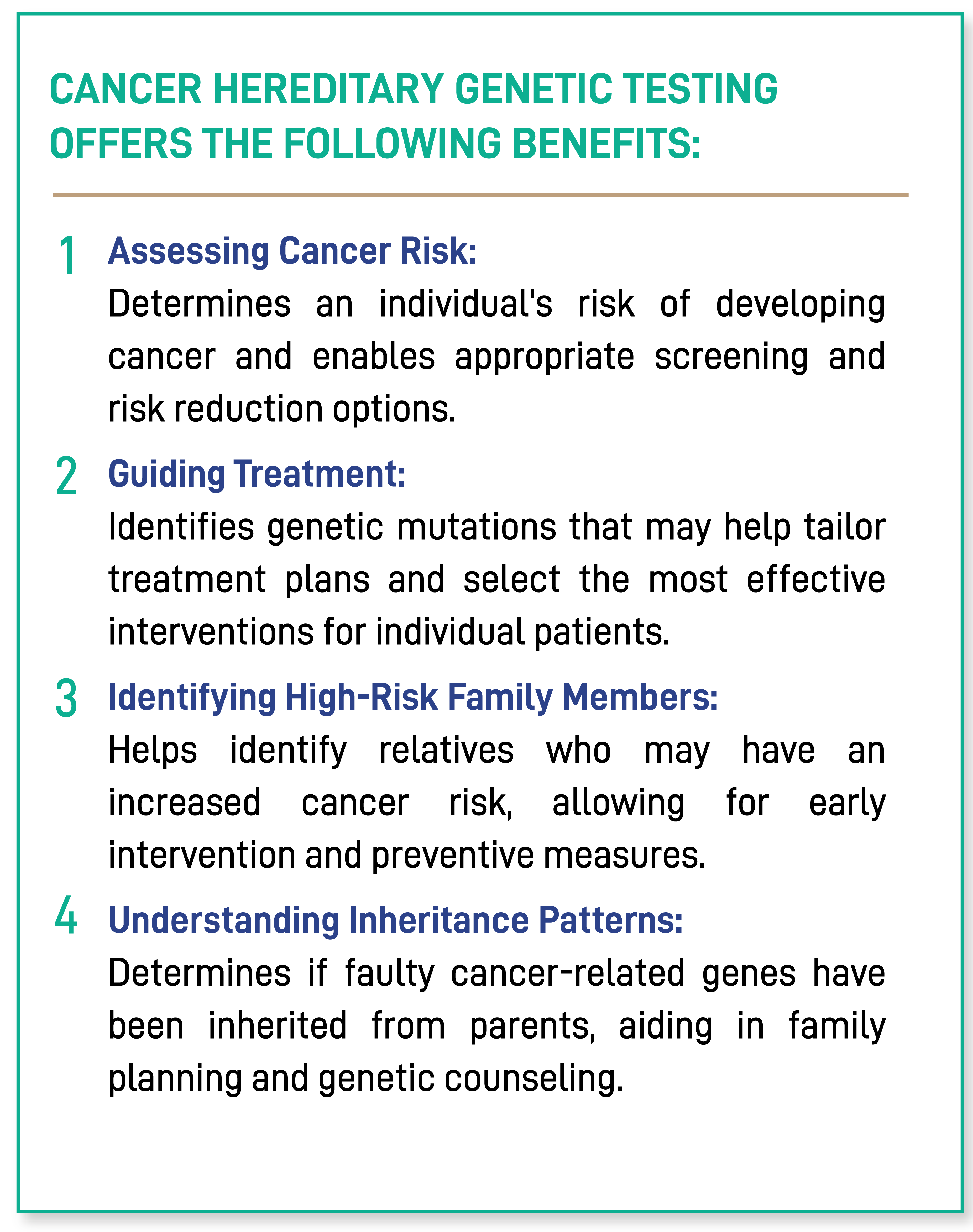
CANCER HEREDITARY GENETIC TESTING OFFERS THE FOLLOWING BENEFITS:
Assesing Cancer Risk:
Determines an individual’s risk of developing cancer and enables appropriate screening and risk reduction options.
Guiding Treatment:
Identifies genetic mutations that may help tailor treatment plans and select the most effective interventions for individual patients.
Identifying High-Risk Family Members:
Helps identify relatives who may have an increased cancer risk, allowing for early intervention and preventive measures.
Understanding Inheritance Patterns:
Determines if faulty cancer-related genes have been inherited from parents, aiding in family planning and genetic counseling.







